| EIF1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | EIF1, A121, EIF-1, ISO1, SUI1, eukaryotic translation initiation factor 1, Eukaryotic initiation factor 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | MGI: 105125; HomoloGene: 130538; GeneCards: EIF1; OMA:EIF1 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Eukaryotic translation initiation factor 1 (eIF1) is a protein that in humans is encoded by the EIF1 gene. It is related to yeast SUI1.[5][6][7]
eIF1 interacts with the eukaryotic small (40S) ribosomal subunit and eIF3, and is a component of the 43S preinitiation complex (PIC).[8] eIF1 and eIF1A bind cooperatively to the 40S to stabilize an "open" conformation of the preinitiation complex (PIC) during eukaryotic translation initiation.[8] eIF1 binds to a region near the ribosomal P-site in the 40S subunit and functions in a manner similar to the structurally related bacterial counterpart IF3.[9]
Structure
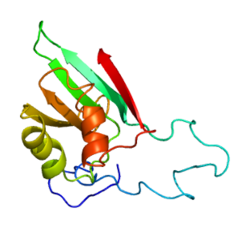
eIF1's structure was first determined in 1999 by solution-state NMR spectroscopy, which revealed that it consists of a five-stranded beta-sheet which is sided by two alpha-helices.[10] Crystallographic experiments showed that eIF1 is located at the P-site of the small ribosomal subunit, binding to the 18S rRNA with a basic surface.[11] To date, a number of cryo-EM structures have been solved that include eIF1 in the context of various translation initiation complexes.[12][13][14]
Function
In eukaryotic cells, translation initiation on an mRNA involves scanning of the mRNA by the 43S pre-initiation complex in search of the translation initiation start codon.[15] Accurate identification of the start codon is very important, as other translation start sites may lead to the production of defect proteins. A codon is detected as a start codon by interaction with the tRNA's anticodon that is positioned in the P-site of the small ribosomal subunit, which leads to closing of the pre-initiation complex.[16] eIF1 is positioned on the small ribosomal subunit such that it blocks the closure of the pre-initiation complex. It thereby aids in selecting the correct start codon, since only the correct codon-anticodon interaction provides enough energy to displace eIF1 and thus close the pre-initiation complex.[17]
See also
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000173812 – Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000035530 – Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ Fields C, Adams MD (January 1994). "Expressed sequence tags identify a human isolog of the suil translation initiation factor". Biochemical and Biophysical Research Communications. 198 (1): 288–91. Bibcode:1994BBRC..198..288F. doi:10.1006/bbrc.1994.1040. PMID 7904817.
- ↑ Sheikh MS, Fernandez-Salas E, Yu M, Hussain A, Dinman JD, Peltz SW, Huang Y, Fornace AJ (June 1999). "Cloning and characterization of a human genotoxic and endoplasmic reticulum stress-inducible cDNA that encodes translation initiation factor 1(eIF1(A121/SUI1))". The Journal of Biological Chemistry. 274 (23): 16487–93. doi:10.1074/jbc.274.23.16487. PMID 10347211.
- ↑ "Entrez Gene: EIF1 eukaryotic translation initiation factor 1".
- 1 2 Aitken CE, Lorsch JR (June 2012). "A mechanistic overview of translation initiation in eukaryotes". Nature Structural & Molecular Biology. 19 (6): 568–76. doi:10.1038/nsmb.2303. PMID 22664984. S2CID 9201095.
- ↑ Fraser CS (July 2015). "Quantitative studies of mRNA recruitment to the eukaryotic ribosome". Biochimie. 114: 58–71. doi:10.1016/j.biochi.2015.02.017. PMC 4458453. PMID 25742741.
- ↑ Fletcher, C.Mark; Pestova, Tatyana V.; Hellen, Christopher U.T.; Wagner, Gerhard (1999). "Structure and interactions of the translation initiation factor eIF1". The EMBO Journal. pp. 2631–2637. doi:10.1093/emboj/18.9.2631. PMC 1171342. PMID 10228174.
- ↑ Rabl, Julius; Leibundgut, Marc; Ataide, Sandro F.; Haag, Andrea; Ban, Nenad (2011). "Crystal Structure of the Eukaryotic 40 S Ribosomal Subunit in Complex with Initiation Factor 1". Science. 331 (6018): 730–736. Bibcode:2011Sci...331..730R. doi:10.1126/science.1198308. hdl:20.500.11850/153130. PMID 21205638.
- ↑ Petrychenko, Valentyn; Yi, Sung-Hui; Liedtke, David; Peng, Bee-Zen; Rodnina, Marina V.; Fischer, Niels (2025). "Structural basis for translational control by the human 48S initiation complex". Nature Structural & Molecular Biology. 32: 62–72. doi:10.1038/s41594-024-01378-4.
- ↑ Brito Querido, Jailson; Sokabe, Masaaki; Díaz-López, Irene; Gordiyenko, Yuliya; Zuber, Philipp; Du, Yifei; Albacete-Albacete, Lucas; Ramakrishnan, V.; Fraser, Christopher S. (2024). "Human tumor suppressor protein Pdcd4 binds at the mRNA entry channel in the 40S small ribosomal subunit". Nature Communications. 15 (1) 6633. Bibcode:2024NatCo..15.6633B. doi:10.1038/s41467-024-50672-8. PMC 11310195. PMID 39117603.
- ↑ Villamayor-Belinchón, Laura; Sharma, Prafful; Gordiyenko, Yuliya; Llácer, Jose L.; Hussain, Tanweer (2024). "Structural basis of AUC codon discrimination during translation initiation in yeast". Nucleic Acids Research. 52 (18): 11317–11335. doi:10.1093/nar/gkae737. PMID 39193907.
- ↑ Hinnebusch, Alan G. (2017). "Structural Insights into the Mechanism of Scanning and Start Codon Recognition in Eukaryotic Translation Initiation". Trends in Biochemical Sciences. 42 (8): 589–611. doi:10.1016/j.tibs.2017.03.004. PMID 28442192.
- ↑ Hussain, Tanweer; Llácer, Jose L.; Fernández, Israel S.; Munoz, Antonio; Martin-Marcos, Pilar; Savva, Christos G.; Lorsch, Jon R.; Hinnebusch, Alan G.; Ramakrishnan, V. (2014). "Structural Changes Enable Start Codon Recognition by the Eukaryotic Translation Initiation Complex". Cell. 159 (3): 597–607. doi:10.1016/j.cell.2014.10.001. PMC 4217140. PMID 25417110.
- ↑ Thakur, Anil; Hinnebusch, Alan G. (2018). "EIF1 Loop 2 interactions with Met-tRNA i control the accuracy of start codon selection by the scanning preinitiation complex". Proceedings of the National Academy of Sciences. 115 (18): E4159 – E4168. Bibcode:2018PNAS..115E4159T. doi:10.1073/pnas.1800938115. PMC 5939108. PMID 29666249.
- ↑ "EIF1 - Eukaryotic translation initiation factor 1 - Homo sapiens (Human) - EIF1 gene & protein". www.uniprot.org. Retrieved 2018-09-23.
Further reading
- Lian Z, Pan J, Liu J, Zhang S, Zhu M, Arbuthnot P, Kew M, Feitelson MA (March 1999). "The translation initiation factor, hu-Sui1 may be a target of hepatitis B X antigen in hepatocarcinogenesis". Oncogene. 18 (9): 1677–87. doi:10.1038/sj.onc.1202470. PMID 10208429. S2CID 22809502.
- Fletcher CM, Pestova TV, Hellen CU, Wagner G (May 1999). "Structure and interactions of the translation initiation factor eIF1". The EMBO Journal. 18 (9): 2631–7. doi:10.1093/emboj/18.9.2631. PMC 1171342. PMID 10228174.
- Chin LS, Singh SK, Wang Q, Murray SF (2000). "Identification of okadaic-acid-induced genes by mRNA differential display in glioma cells". Journal of Biomedical Science. 7 (2): 152–9. doi:10.1007/BF02256622. PMID 10754390.
- Mendell JT, Medghalchi SM, Lake RG, Noensie EN, Dietz HC (December 2000). "Novel Upf2p orthologues suggest a functional link between translation initiation and nonsense surveillance complexes". Molecular and Cellular Biology. 20 (23): 8944–57. doi:10.1128/MCB.20.23.8944-8957.2000. PMC 86549. PMID 11073994.
- Rush J, Moritz A, Lee KA, Guo A, Goss VL, Spek EJ, Zhang H, Zha XM, Polakiewicz RD, Comb MJ (January 2005). "Immunoaffinity profiling of tyrosine phosphorylation in cancer cells". Nature Biotechnology. 23 (1): 94–101. doi:10.1038/nbt1046. PMID 15592455. S2CID 7200157.
External links
- EIF1 human gene location in the UCSC Genome Browser.
- EIF1 human gene details in the UCSC Genome Browser.
- Cap-dependent translation initiation from Nature Reviews Microbiology. A good image and overview of the function of initiation factors
- Overview of all the structural information available in the PDB for UniProt: P41567 (Eukaryotic translation initiation factor 1) at the PDBe-KB.




